Photo from wikipedia
Abstract Cerium oxide makes one of the most promising materials for chemical transformations in environmental and energy applications. Herein, the influence of hydrothermal conditions on the physico-chemical characteristics of cerium… Click to show full abstract
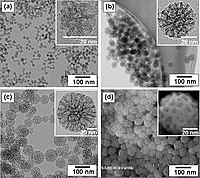
Abstract Cerium oxide makes one of the most promising materials for chemical transformations in environmental and energy applications. Herein, the influence of hydrothermal conditions on the physico-chemical characteristics of cerium oxide prepared from salt solution via ammonia precipitation is analysed. The systems are well characterised using SEM, TEM, XRD analysis, photoluminescence spectra, Raman spectra, TPR study. and XPS analysis. Normal aqueous conditions lead to particles of size ∼ 8 nm, with truncated octahedral geometry, closer to spheroid shape (RT-Ce) bound by {111} and {100} planes. Elevated temperature facilitated preferential exposed {100} plane bounded cubic ceria structures of size ∼15 nm (HT-Ce), which are stabilised by more number of anion vacancies. Low temperature synthesis yielded smaller sized particles with less crystallinity and higher surface area, when compared to hydrothermal route. Lattice defects, represented in terms of Ce3+ ions and associated lattice oxygen vacancies are seen in higher amounts in ceria synthesised via hydrothermal path, as supported by various characterisation results. CeO2 achieved via hydrothermal path exhibited higher catalytic oxidation activity. The catalytic activity of the ceria samples is examined using a model oxidation reaction, vis., CO oxidation. The enhanced activity of HT-Ce is explained through the defect structure induced facile redox shift in the system.
Journal Title: Journal of Solid State Chemistry
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!