Photo from wikipedia
In this work, we developed novel core-shell nanoparticle systems with magnetic core and polymer shell via atom transfer radical polymerization for use as high affinity nanoadsorbents for organic contaminants in… Click to show full abstract
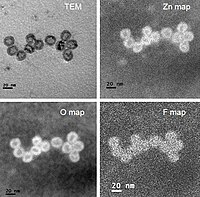
In this work, we developed novel core-shell nanoparticle systems with magnetic core and polymer shell via atom transfer radical polymerization for use as high affinity nanoadsorbents for organic contaminants in water and wastewater treatment. Polyphenolic-based moieties, curcumin multiacrylate (CMA) and quercetin multiacrylate (QMA), were incorporated into poly(ethylene glycol) (PEG) based polymeric shells to create high affinity binding sites for the capture of polychlorinated biphenyls (PCBs) as a model pollutant. The resulting magnetic nanoparticles (MNPs) were characterized by Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), transmission electron microscopy (TEM), X-ray diffraction (XRD), dynamic light scattering (DLS), and UV-visible spectroscopy. The affinity of these novel materials for PCB 126 was evaluated and fitted to the nonlinear Langmuir model to determine binding affinities (KD). The KD values obtained were: PEG MNPs (8.42 nM) < IO MNPs (8.23nM) < QMA MNPs (5.88 nM) < CMA MNPs (2.72 nM), demonstrating that the presence of polyphenolic-based moieties enhanced PCB 126 binding affinity, which is hypothesized to be a result of π - π stacking interactions. These values are lower that KD values for activated carbon, providing strong evidence that these novel core-shell nanoparticles have a promising application as nanoadsorbents for specific organic contaminants offering a cost effective alternative to current remediation approaches.
Journal Title: Materials chemistry and physics
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!