Photo from wikipedia
Phosphatidylcholine (PC) is a rare membrane lipid in bacteria but crucial for virulence of various plant and animal pathogens. The pcs- mutant lacking PC in bacterial membranes of Pseudomonas syringae… Click to show full abstract
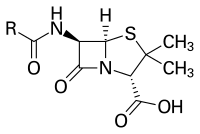
Phosphatidylcholine (PC) is a rare membrane lipid in bacteria but crucial for virulence of various plant and animal pathogens. The pcs- mutant lacking PC in bacterial membranes of Pseudomonas syringae pv. syringae van Hall 1336 displayed more ampicillin resistance. Ampicillin susceptibility tests gave an IC50 (half maximal inhibitory concentration) of 52 mg/ml for Pseudomonas syringae pv. syringae van Hall 1336, 53 mg/ml for the complemented strain 1336 RM (pcs-/+) and 90 mg/ml for the 1336 pcs- mutant. Activity assay of β-lactamase in periplasmic extracts gave 0.050 U/mg for the 1336 wild type, 0.052 U/mg for the 1336RM (pcs-/+), 0.086 U/mg for the 1336 pcs- mutant. Analysis by western blotting showed that the content of AmpC enzyme was markedly different in periplasmic extracts between the wild-type and pcs- mutant strains. Reverse transcriptase PCR also showed that the presence or absence of PC in bacterial membranes did not affect the transcription of ampC gene. The phenotype of the pcs- mutant was able to be recovered to the wild type by introducing a wild-type pcs gene into the pcs- mutant. Similar results were also obtained from the soil-dwelling bacterium Pseudomonas sp. 593. Our results demonstrate that the absence of PC in bacterial membranes facilitates the translocation of Sec-dependent β-lactamase AmpC from cytoplasm to periplasm, and the enhanced ampicillin-resistance in the pcs- strains mainly comes from effective translocation of AmpC via Sec-pathway.
Journal Title: Microbial pathogenesis
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!