Photo from wikipedia
Abstract By dynamic laser method, the solubilities of succinic acid, glutaric acid and adipic acid in propionic acid + e-caprolactone mixtures and propionic acid + cyclohexanone mixtures were determined under atmospheric pressure. The experimental… Click to show full abstract
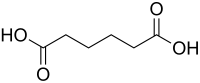
Abstract By dynamic laser method, the solubilities of succinic acid, glutaric acid and adipic acid in propionic acid + e-caprolactone mixtures and propionic acid + cyclohexanone mixtures were determined under atmospheric pressure. The experimental temperature ranged from 283.95 to 340.45 K, and the mass fraction of propionic acid in the solvent mixtures ranged from 0.00 to 1.00 respectively. It was found that when the solvent composition is constant, the solubilities of succinic acid, glutaric acid and adipic acid would increases with the increase of the temperature. What is more, at constant temperature, with the mass fraction of propionic acid in mixtures increasing, the solubilities of glutaric acid and adipic acid increase gradually, on the contrary, the solubility of succinic acid decrease gradually. The experimental data were correlated by the modified Apelblat equation and the modified nonrandom two-liquid (NRTL) activity coefficient models. The maximum value of average relative deviation was 3.015%, which shows the values of the solubility calculated showed good agreement with the experimental observations. Furthermore, the thermodynamic functions including dissolution enthalpy, entropy and Gibbs energy were obtained from the solubility data by using the van't Hoff equation.
Journal Title: Journal of Molecular Liquids
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!