Photo from wikipedia
Abstract The ionic liquids have great potential in the field of energy and power engineering for their thermophysical properties can be modified by changing the types of anion and cation.… Click to show full abstract
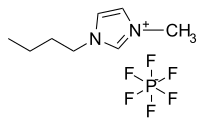
Abstract The ionic liquids have great potential in the field of energy and power engineering for their thermophysical properties can be modified by changing the types of anion and cation. In this paper, molecular dynamics simulations are employed to investigate the molecular characteristics of 1-Butyl-3-methyl tetra-fluoroborate [BMIM][BF4] ionic liquid and its blends with water on the Pt surface. The results show that the number of [BMIM][BF4] ionic liquid near Pt surface slightly decreases with the molar ratio of water molecules increasing. And mobility of anions and cations of [BMIM][BF4] ionic liquid are strengthened by the addition of water. Additionally, the thermal conductivity and viscosity of [BMIM][BF4] ionic liquid are weakened with water blended. A hypothesis that water replacing the [BF4]− to form the hydrogen bond with [BMIM]+ is proposed to explain the weakening of viscosity of the mixture system. And the Pt surface will strengthen the viscosity and thermal conductivity of [BMIM][BF4]/water mixtures in the vertical direction of the substrate.
Journal Title: Journal of Molecular Liquids
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!