Photo from wikipedia
Abstract Synthesis, spectral and X-ray analysis of three compounds, i.e. 3β-acetoxy-12-hydroxyimino-18β-oleanan-28,13β-olide (substrate) and 3β-acetoxy-12-nitrile-12,13-seco-15(14 → 13)-abeoolean-14(27)-en-28,13β-olide and 3β-acetoxy-12-oxo-12a-aza-C-homoolean-13(18)-en-28-oic acid (Beckmann rearrangement reaction products) are described. Structural analysis revealed that the oxime group… Click to show full abstract
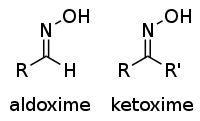
Abstract Synthesis, spectral and X-ray analysis of three compounds, i.e. 3β-acetoxy-12-hydroxyimino-18β-oleanan-28,13β-olide (substrate) and 3β-acetoxy-12-nitrile-12,13-seco-15(14 → 13)-abeoolean-14(27)-en-28,13β-olide and 3β-acetoxy-12-oxo-12a-aza-C-homoolean-13(18)-en-28-oic acid (Beckmann rearrangement reaction products) are described. Structural analysis revealed that the oxime group in the ring C in substrate molecule had an E-configuration. The nitrile product with retained lactone group was a result of major transformations within rings C and D of oleanane skeleton. In lactam product free carboxyl group and a double bond in ring D instead of lactone system were formed in Beckmann rearrangement reaction.
Journal Title: Journal of Molecular Structure
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!