Photo from wikipedia
Abstract The optimized geometry and vibrational frequencies of an organic compound copper phthalocyanine tetrasulfonic acid tetra sodium salt (CuPcTS) have been investigated using density functional theory. We have also optimized… Click to show full abstract
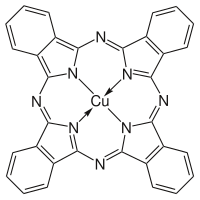
Abstract The optimized geometry and vibrational frequencies of an organic compound copper phthalocyanine tetrasulfonic acid tetra sodium salt (CuPcTS) have been investigated using density functional theory. We have also optimized the structures of copper phthalocyanine (CuPc) and its substituted complex and simulated their vibrational spectra to study the substitution effects of sulfonic acid group at the peripheral sites of copper phthalocyanine. Experimentally, vibrational frequencies of these molecules have been studied using infrared absorption, Raman spectroscopic techniques. It has been found that the vibrational frequencies obtained from the theoretical studies generally agree with the experiment. Most of the bands show red shift in CuPcTS due to decrease of stretching force constant of C C and reduction in the contribution from in-plane C H bending vibrations. New bands have been observed due to S O vibrations. Degeneracy of IR bands is removed because of change of symmetry from D4h in CuPc to C1 in substituted CuPc. Effect of substitution on the structural parameters and mode compositions of the vibrational bands have also been studied. The electronic absorption spectra of the substituted copper phthalocyanine in different solvents have been studied using UV–Vis spectroscopy. It has been found that the substituted copper phthalocyanine shows aggregation effect in water because of greater extent of hydrogen bonding. The direct optical band gap energy and refractive index of CuPcTS have also been calculated in the different solvents. We have also plotted the I V characteristic for both the molecules and it has been found that electrical and photoconductivity of CuPcTS is more as compared to CuPc. The intermolecular charge transfer interactions have been studied by HOMO-LUMO and natural bonding orbital (NBO) analysis. The values of first order hyperpolaizability for both the molecules indicate that substituted copper phthalocyanine acquires excellent non-linear optical (NLO) properties. The interaction sites of the both the molecules have been studied by the molecular electrostatic potential (MEP) and solvent accessible surface area (SASA) analysis.
Journal Title: Journal of Molecular Structure
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!