Photo from wikipedia
Abstract The corrosion inhibition of three triazole derivatives; p-tolyl-1H-1,2,3-triazol-5-ol (Z1), 4-Chlorophenyl-1H-1, 2, 3-triazol-5-ol (Z2) and Methyl-4-(5-hydroxy-1H-1, 2, 3-triazol-1-yl) benzoate (Z3) towards Aluminium metal in 1.0 M HCl have been investigated… Click to show full abstract
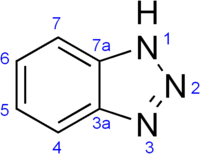
Abstract The corrosion inhibition of three triazole derivatives; p-tolyl-1H-1,2,3-triazol-5-ol (Z1), 4-Chlorophenyl-1H-1, 2, 3-triazol-5-ol (Z2) and Methyl-4-(5-hydroxy-1H-1, 2, 3-triazol-1-yl) benzoate (Z3) towards Aluminium metal in 1.0 M HCl have been investigated using potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), scanning electron microscopy (SEM), B3LYP Density Functional Theory (DFT) and adsorption annealing simulations. The electrochemical findings exhibit an inhibition efficiency increase in the order: Z3 > Z2 > Z1 with a maximum inhibition efficiency (74.7%) obtained at 500 ppm from Z3. Both anodic (βa) and cathodic (βc) Tafel constants was significantly affected by adding inhibitors to the corrosion medium proposing a mixed type nature. The adsorption of the inhibitors on the metal surface was found to be a spontaneous process and obeyed Langmuir isotherm. The conformational scan along with annealing adsorption simulations showed a dihedral angle between the triazole ring and the phenyl ring of only 30 degrees which promotes optimal surface coverage. Global reactivity descriptors, Fukui indices, atomic charges and NBO analysis showed that the priority of charge reallocation form inhibitor MOs to the metal surface is easier in the order: Z3 > Z2 >Z1 which reveal good agreement to the electrochemical measurements. Based on DFT results, an inhibition mechanism was proposed.
Journal Title: Journal of Molecular Structure
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!