Photo from wikipedia
BACKGROUND Treatment of low-risk patients with isolated symptomatic distal deep vein thrombi (IDDVT) is uncertain. OBJECTIVE assess whether two weeks of therapeutic anticoagulation is efficacious/safe for IDDVT. PRIMARY OUTCOME symptomatic… Click to show full abstract
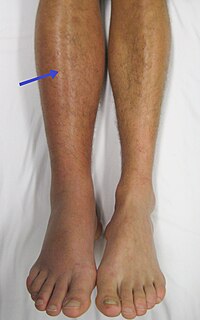
BACKGROUND Treatment of low-risk patients with isolated symptomatic distal deep vein thrombi (IDDVT) is uncertain. OBJECTIVE assess whether two weeks of therapeutic anticoagulation is efficacious/safe for IDDVT. PRIMARY OUTCOME symptomatic three-month venous thromboembolism (VTE) incidence in the two-week anticoagulation group. Secondary outcomes included post-thrombotic syndrome (PTS) and bleeding. METHODS Prospective multicentre cohort study. Consecutive low-risk IDDVT patients enrolled within 72 h of diagnosis and treated with therapeutic dose enoxaparin or rivaroxaban. At two weeks, patients had repeat complete whole leg compression ultrasound (CUS)/clinical review. If resolution of leg symptoms AND no radiological evidence of thrombus extension, anticoagulation was stopped. If ongoing symptoms and/or radiographic extension within distal veins, anticoagulation was continued for four more weeks. Patients with extension into the popliteal vein on two-week ultrasound were treated off-study. Patients were reviewed at three and six months. FINDINGS/INTERPRETATION 241 eligible patients received ≥2 weeks anticoagulation. 167/241 (69%) were assigned to the 2-week anticoagulation group; 71/241 (30%) to the six-week anticoagulation group; 3/241 patients (1%) had extension into the popliteal vein on two-week CUS. Two patients in the two-week anticoagulation group had symptomatic IDDVT recurrence in ≤3 months; VTE recurrence 2/156; 1.3%(95% CI 0.05-4.85%). 69% of patients had complete resolution of symptoms within two weeks. Six-month PTS rates were 8/184, 4.4%(95% CI 2.1-8.5%). No major bleeding was reported. Our findings suggest it's safe/efficacious to stop therapeutic anticoagulation at two weeks in low-risk IDDVT patients with resolution of symptoms/no extension on ultrasound. This could replace 6-12 weeks of anticoagulation for ambulatory, low-risk IDDVT patients. TRIAL REGISTRATION ClinicalTrials.govNCT01252420.
Journal Title: Thrombosis research
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!