Photo from wikipedia
Cardiac myosin binding protein-C (cMyBP-C) is an essential regulatory protein required for proper systolic contraction and diastolic relaxation. We previously showed that N'-terminal domains of cMyBP-C stimulate contraction by binding… Click to show full abstract
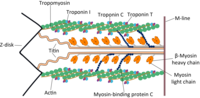
Cardiac myosin binding protein-C (cMyBP-C) is an essential regulatory protein required for proper systolic contraction and diastolic relaxation. We previously showed that N'-terminal domains of cMyBP-C stimulate contraction by binding to actin and activating the thin filament in vitro. In principle, thin filament activating effects of cMyBP-C could influence contraction and relaxation rates, or augment force amplitude in vivo. cMyBP-C binding to actin could also contribute to an internal load that slows muscle shortening velocity as previously hypothesized. However, the functional significance of cMyBP-C binding to actin has not yet been established in vivo. We previously identified an actin binding site in the regulatory M-domain of cMyBP-C and described two missense mutations that either increased (L348P) or decreased (E330K) binding affinity of recombinant cMyBP-C N'-terminal domains for actin in vitro. Here we created transgenic mice with either the L348P or E330K mutations to determine the functional significance of cMyBP-C binding to actin in vivo. Results showed that enhanced binding of cMyBP-C to actin in L348P-Tg mice prolonged the time to end-systole and slowed relaxation rates. Reduced interactions between cMyBP-C and actin in E330K-Tg mice had the opposite effect and significantly shortened the duration of ejection. Neither mouse model displayed overt systolic dysfunction, but L348P-Tg mice showed diastolic dysfunction presumably resulting from delayed relaxation. We conclude that cMyBP-C binding to actin contributes to sustained thin filament activation at the end of systole and during isovolumetric relaxation. These results provide the first functional evidence that cMyBP-C interactions with actin influence cardiac function in vivo.
Journal Title: Journal of molecular and cellular cardiology
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!