Photo from wikipedia
Abstract Developing highly efficient and stable water oxidation catalysts based on abundant metallic elements is a challenge that must be met to fulfill the promise of water splitting for clean… Click to show full abstract
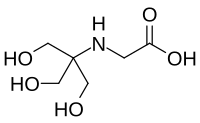
Abstract Developing highly efficient and stable water oxidation catalysts based on abundant metallic elements is a challenge that must be met to fulfill the promise of water splitting for clean energy production. In this work, we developed an oxygen evolution reaction catalyst consisting of a nanostructured film electrodeposited from a phosphate buffer solution (0.2 mol/L, pH = 12.0) containing Cu-tricine complex. A Tafel plot showed that the required overpotential for a current density of 1.0 mA/cm2 was only 395 mV and the Tafel slope was 46.7 mV/decade. In addition, the Cu-tricine film maintained a stable current density of 7.5 mA/cm2 for the oxygen evolution reaction in phosphate buffer solution for 10 h, and a Faradaic efficiency of 99% was obtained.
Journal Title: Chinese Journal of Catalysis
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!