Photo from wikipedia
Biothiols and SO2 derivatives, as essential reactive sulfur species (RSS), play vital roles in various physiological processes and have a close network of generation and metabolic pathways among them. To… Click to show full abstract
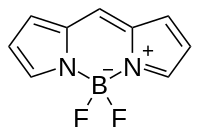
Biothiols and SO2 derivatives, as essential reactive sulfur species (RSS), play vital roles in various physiological processes and have a close network of generation and metabolic pathways among them. To clarify their complex correlations, fluorescent probes to simultaneously detect GSH, Cys, and SO2 derivatives are highly desirable. Herein, we develop the first fluorescent probe (BO-HEM) to simultaneously discriminate GSH, Cys, and SO2 derivatives. The fluorescent probe is designed by integration of hemicyanine and BODIPY fluorophores through an ether bond. The ether bond of the probe is rapidly replaced by thiolates through nucleophilic aromatic substitution (SNAr) to generate hemicyanine with NIR fluorescence and sulfur-BODIPY. The amino groups of Cys but not GSH then further replace the thiolate to form amino-BODIPY. As for SO32–, nucleophilic addition to the double bond of BO-HEM generates adduct O-BODIPY with green fluorescence. To further improve the sensing performance, the nanoprobe with increased reactivity and biocompatibility is constructed by encapsulation of BO-HEM into the polymeric micelle. More importantly, by taking advantage of the hydrophilicity of the reaction products, the spectral discrimination was further enhanced to avoid signal interference. The nanoprobe is applied to discriminate biothiols and SO2 derivatives in living cells through three-color fluorescence imaging.
Journal Title: Analytical Chemistry
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!