Photo from wikipedia
Aqueous mixtures of two or more surfactants are often employed for research or industrial purposes because such mixtures offer advantages over single-surfactant systems. This is particularly true for mixtures of… Click to show full abstract
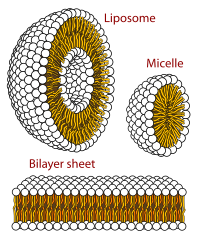
Aqueous mixtures of two or more surfactants are often employed for research or industrial purposes because such mixtures offer advantages over single-surfactant systems. This is particularly true for mixtures of fluorocarbon (FC) and hydrocarbon (HC) surfactants, which display a broad range of mutual miscibilities in mixed micelles. Unfortunately, the prediction and even the experimental elucidation of the micellar mixing behavior of surfactant mixtures remain challenging, as evidenced by conflicting results and conclusions derived from diverse, and often complex, mixing models. One of the most intriguing questions is whether certain combinations of FC and HC surfactants form only one type of mixed micelle or rather demix into two micelle populations, namely, FC-rich and HC-rich ones. Here, we demonstrate a novel approach to the model-free analysis of critical micellar concentrations (CMCs) of surfactant mixtures that is based on a fit of the experimental data with cubic splines using a stringent thermodynamic criterion for mixing. As a proof of principle, we analyze CMC values determined by isothermal titration calorimetry and confirm the conclusions with the aid of combined 1H- and 19F-NMR spectroscopy. Specifically, we show that aqueous mixtures of an FC maltoside and an HC maltoside conform with the assumption of only one type of micelle regardless of the mixing ratio, whereas combining the same FC surfactant with an HC surfactant carrying a zwitterionic phosphocholine headgroup gives rise to two coexisting micelle populations at high mole fractions of the FC maltoside.
Journal Title: Analytical chemistry
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!