Photo from wikipedia
It is generally recognized that phenol-containing molecules mainly undergo phase II metabolic reactions, whereas glucuronide and sulfate are conjugated to form water-soluble products. Here, we report direct reactions of phenolic… Click to show full abstract
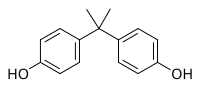
It is generally recognized that phenol-containing molecules mainly undergo phase II metabolic reactions, whereas glucuronide and sulfate are conjugated to form water-soluble products. Here, we report direct reactions of phenolic pollutants (triclosan, alkylphenol, bisphenol A [BPA], and its analogues) and some endogenous metabolites (vitamin E [VE] and estradiol) to generate new lipophilic ether products (e.g., BPA-O-VEs and alkylphenol-O-estradiol). A nontargeted screening strategy was used to identify the products in human liver microsome incubations, and the most abundant products (BPA-O-VEs) were confirmed via in vivo exposure in mice. BPA-O-VEs were frequently detected in sera from the general population at levels comparable to those of glucuronide/sulfate-conjugated BPA. Recombinant human cytochrome P450s were applied to find that CYP3A4 catalyzed the formation of these newly discovered ether metabolites by linking the VE hydroxyl group to the BPA phenolic ring, leading to the significantly reduced antioxidative activities of BPA-O-VEs compared to VEs. The effects of the reaction on the homeostasis of reacted biomolecules were finally assessed by in vitro assay and in vivo mice exposures. The generation of BPA-O-VEs decreased the VE concentrations and increased the reactive oxygen species generation after exposure to BPA at environmentally relevant concentrations. The identified reactions provided an overlooked metabolic disruption pathway for phenolic pollutants.
Journal Title: Environmental science & technology
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!