Photo from wikipedia
Up to now, uranium dioxide, the most used nuclear fuel, was said to have a Fm3̅m crystalline structure from 30 to 3000 K, and its behavior was modeled under this… Click to show full abstract
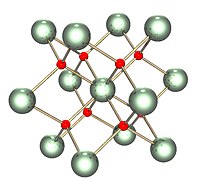
Up to now, uranium dioxide, the most used nuclear fuel, was said to have a Fm3̅m crystalline structure from 30 to 3000 K, and its behavior was modeled under this assumption. However, recently X-ray diffraction experiments provided atomic pair-distribution functions of UO2, in which UO distance was shorter than the expected value for the Fm3̅m space group. Here we show neutron diffraction results that confirm this shorter UO bond, and we also modeled the corresponding pair-distribution function showing that UO2 has a local Pa3̅ symmetry. The existence of a local lower symmetry in UO2 could explain some unexpected properties of UO2 that would justify UO2 modeling to be reassessed. It also deserves more study from an academic point of view because of its good thermoelectric properties that may originate from its particular crystalline structure.
Journal Title: Inorganic chemistry
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!