Photo from wikipedia
The iron(II) complexes of two structural isomers of 2-(1 H-imidazol-2-yl)diazine reveal how ligand design can be a successful strategy to control the electronic and magnetic properties of complexes by fine-tuning… Click to show full abstract
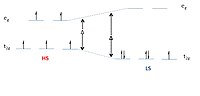
The iron(II) complexes of two structural isomers of 2-(1 H-imidazol-2-yl)diazine reveal how ligand design can be a successful strategy to control the electronic and magnetic properties of complexes by fine-tuning their ligand field. The two isomers only differ in the position of a single diazinic nitrogen atom, having either a pyrazine (Z) or a pyrimidine (M) moiety. However, [Fe(M)3](ClO4)2 is a spin-crossover complex with a spin transition at 241 K, whereas [Fe(Z)3](ClO4)2 has a stable magnetic behavior between 2 and 300 K. This is corroborated by temperature-dependent Mössbauer spectra showing the presence of a quintet and a singlet state in equilibrium. The temperature-dependent single-crystal X-ray diffraction results relate the spin-crossover observed in [Fe(M)3](ClO4)2 to changes in the bond distances and angles of the coordination sphere of iron(II), hinting at a stronger σ donation of ligand Z in comparison to ligand M. The UV/vis spectra of both complexes are solved by means of the multiconfigurational wave-function-based method CASPT2 and confirm their different spin multiplicities at room temperature, as observed in the Mössbauer spectra. Calculations show larger stabilization of the singlet state in [Fe(Z)3]2+ than in [Fe(M)3]2+, stemming from the slightly stronger ligand field of the former (506 cm-1 in the singlet). This relatively weak effect is indeed capable of changing the spin multiplicity of the complexes and causes the appearance of the spin transition in the M complex.
Journal Title: Inorganic chemistry
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!