Photo from wikipedia
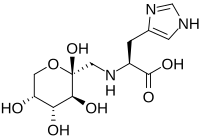
N-(1-Deoxy-d-fructos-1-yl)-histidine (Fru-His), one of the Amadori compounds, widely presents in processed foods, and its potential functional activities have attracted extensive attention in recent years. In this work, the angiotensin-converting enzyme… Click to show full abstract
N-(1-Deoxy-d-fructos-1-yl)-histidine (Fru-His), one of the Amadori compounds, widely presents in processed foods, and its potential functional activities have attracted extensive attention in recent years. In this work, the angiotensin-converting enzyme (ACE) inhibitory activity and mechanism of Fru-His were investigated. The IC50 value of Fru-His was 0.150 ± 0.019 mM, and there was no obvious degradation of Fru-His after digestion simulation, showing that Fru-His has good ACE inhibition and digestive stability. Fru-His was a competitive inhibitor according to the enzyme inhibition kinetic analysis. The interaction between ACE and Fru-His occurred spontaneously mainly through hydrogen bonding, and the process was accompanied by fluorescence quenching and the alteration of the secondary structure of ACE. The molecular docking data supported the above results. Fru-His was attached to ACE's S1 active pocket through hydrogen bonds and interacted with zinc ions in active sites. The present study demonstrates that food-derived Fru-His has the potential to relieve hypertension.
Journal Title: Journal of agricultural and food chemistry
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!