Photo from wikipedia
Although various inhibitors have been employed to react with phenylacetaldehyde to form adducts and thus interrupt the formation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), high concentrations of PhIP remain in the final system.… Click to show full abstract
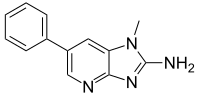
Although various inhibitors have been employed to react with phenylacetaldehyde to form adducts and thus interrupt the formation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), high concentrations of PhIP remain in the final system. It remains unknown whether other critical aldehyde or ketone intermediates are involved in the generation of PhIP, and scavenging these reactive carbonyls simultaneously may achieve higher inhibitory efficiency of PhIP. In this study, reactive carbonyls in a glucose/creatinine/phenylalanine model system were first identified by gas chromatography-mass spectrometry (GC-MS), and then the single and synergistic effects of nonprecursor amino acids (cysteine, methionine, proline, histidine, arginine, and leucine) on scavenging reactive carbonyls were investigated to find out promising combination partners. The obtained results showed that the concentrations of benzaldehyde and phenylacetaldehyde in the glucose/creatinine/phenylalanine model system reached 0.49 ± 0.01 and 6.22 ± 0.21 μg/mL, respectively. Heating these carbonyl compounds in the presence of creatinine resulted in the quantity of PhIP produced increasing linearly with the added quantity of benzaldehyde (r = 0.9733, P = 0.0002) and phenylacetaldehyde (r = 0.9746, P = 0.0002), indicating that both compounds are key intermediates for PhIP generation. Among the investigated amino acids, histidine produced the maximum inhibition of PhIP formation (78-99%) in the benzaldehyde/creatinine model system, and proline produced the maximum inhibition of PhIP formation (13-97%) in the phenylacetaldehyde/creatinine model system, where both compounds decreased PhIP formation in a dose-dependent manner. Histidine in combination with proline enhanced the inhibitory effect against PhIP formation at a low addition level, where the highest inhibitory efficiency was obtained using a 1:3 mass ratio of histidine to proline (2 mg/mL in total), reducing PhIP formation by 96%. These findings suggest that histidine-proline combinations can scavenge benzaldehyde and phenylacetaldehyde simultaneously, enhancing the suppression of PhIP formation.
Journal Title: Journal of agricultural and food chemistry
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!