Photo from wikipedia
The rapid increase in bacterial resistance requires an urgent need for developing new antimicrobial agents to combat multidrug-resistant pathogens. In this study, we designed and synthesized a series of kaempferol… Click to show full abstract
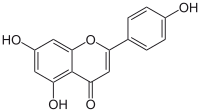
The rapid increase in bacterial resistance requires an urgent need for developing new antimicrobial agents to combat multidrug-resistant pathogens. In this study, we designed and synthesized a series of kaempferol derivatives as antimicrobial agents biomimicking the structural properties and biological functions of Host Defense Peptides (HDPs). After fine-tuning of hydrophobic and cationic hydrophilic moieties linked to the flavone scaffold of kaempferol, we obtained a lead compound (52) that displayed high membrane selectivity (> 128), poor hemolytic activity, low cytotoxicity to mammalian cells, and excellent activity against Gram-positive bacteria (MICs = 1.56 µg/mL), including MRSA. Compound 52 can kill bacteria quickly by destroying the bacterial membranes, and avoid developing bacterial resistance. Moreover, compound 52 exhibited potent in vivo antibacterial activity against Staphylococcus aureus in a murine corneal infection model. These results indicated that compound 52 had therapeutic potential as a novel membrane-active antimicrobial to combat Gram-positive bacterial infections.
Journal Title: Journal of medicinal chemistry
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!