Photo from wikipedia
Visceral leishmaniasis is responsible for up to 30,000 deaths every year. Current treatments have shortcomings that include toxicity and variable efficacy across endemic regions. Previously, we reported the discovery of… Click to show full abstract
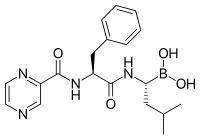
Visceral leishmaniasis is responsible for up to 30,000 deaths every year. Current treatments have shortcomings that include toxicity and variable efficacy across endemic regions. Previously, we reported the discovery of GNF6702, a selective inhibitor of the kinetoplastid proteasome, which cleared parasites in murine models of leishmaniasis, Chagas disease, and human African trypanosomiasis. Here, we describe the discovery and characterization of LXE408, a structurally related kinetoplastid-selective proteasome inhibitor currently in Phase 1 human clinical trials. Furthermore, we present high-resolution cryo-EM structures of the Leishmania tarentolae proteasome in complex with LXE408, which provides a compelling explanation for the noncompetitive mode of binding of this novel class of inhibitors of the kinetoplastid proteasome.
Journal Title: Journal of Medicinal Chemistry
Year Published: 2020
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!