Photo from wikipedia
A series of benzyloxy and phenoxy derivatives of the adenosine receptor agonists N6-cyclopentyl adenosine (CPA) and N6-cyclopentyl 5′-N-ethylcarboxamidoadenosine (CP-NECA) were synthesized, and their potency and selectivity were assessed. We observed… Click to show full abstract
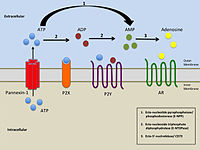
A series of benzyloxy and phenoxy derivatives of the adenosine receptor agonists N6-cyclopentyl adenosine (CPA) and N6-cyclopentyl 5′-N-ethylcarboxamidoadenosine (CP-NECA) were synthesized, and their potency and selectivity were assessed. We observed that the most potent were the compounds with a halogen in the meta position on the aromatic ring of the benzyloxy- or phenoxycyclopentyl substituent. In general, the NECA-based compounds displayed greater A1R selectivity than the adenosine-based compounds, with N6-2-(3-bromobenzyloxy)cyclopentyl-NECA and N6-2-(3-methoxyphenoxy)cyclopentyl-NECA showing ∼1500-fold improved A1R selectivity compared to NECA. In addition, we quantified the compounds’ affinity and kinetics of binding at both human and rat A1R using a NanoBRET binding assay and found that the halogen substituent in the benzyloxy- or phenoxycyclopentyl moiety seems to confer high affinity for the A1R. Molecular modeling studies suggested a hydrophobic subpocket as contributing to the A1R selectivity displayed. We believe that the identified selective potent A1R agonists are valuable tool compounds for adenosine receptor research.
Journal Title: Journal of Medicinal Chemistry
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!