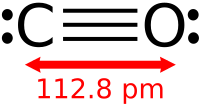
Photo from wikipedia
The ability to control materials stability, bonding, and transformation by thermo-mechanical and chemical means is significant for development of high-energy-density extended solids. We report that doping hydrogen (∼10%) in carbon… Click to show full abstract
The ability to control materials stability, bonding, and transformation by thermo-mechanical and chemical means is significant for development of high-energy-density extended solids. We report that doping hydrogen (∼10%) in carbon monoxide (CO) can greatly lower the polymerization pressure of CO and enhance the stability of recovered polymeric CO products at ambient conditions. Hydrogen-doped CO crystallizes into well-grown dendrites of β-CO-like phase at 3.2 GPa, which polymerizes to highly unsaturated black polymer (phase I) at ∼4.7 (5.8) GPa. Upon further compression, this highly colored polymer transforms into a translucent 3D network structure (phase II) at 6–7 (10–17) GPa and then a transparent 2D layer structure (phase III) at 20–30 (30–60) GPa. A similar series of transformations are also found in pure CO but at considerably higher transition pressures, as noted in parentheses. All polymeric phases are recoverable at ambient conditions, exhibiting an array of phase stability and novel properties s...
Journal Title: Journal of Physical Chemistry C
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!