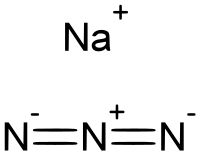
Photo from wikipedia
Amides reacted with NaN3 to give the acyl azides in DMF at 25 °C and produce the symmetrical ureas in THF/H2O at 80 °C via the sequential reaction of acyl… Click to show full abstract
Amides reacted with NaN3 to give the acyl azides in DMF at 25 °C and produce the symmetrical ureas in THF/H2O at 80 °C via the sequential reaction of acyl substitution and Curtius rearrangement. All acyl azides were also obtained from the secondary amides via sequential reaction of p-toluenesulfonyl chloride and NaN3. In addition, keto-stabilized iminophosphoranes were prepared from a one-pot reaction of amides, NaN3, and phosphines.
Journal Title: Organic letters
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!