Photo from wikipedia
Oxide-derived copper (OD-Cu) has been shown to favor C2 and C3 products in electrochemical CO reduction at lower overpotentials than polycrystalline copper (Cu-poly). Despite numerous studies and proposed mechanisms, the… Click to show full abstract
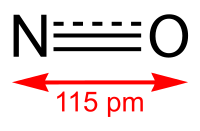
Oxide-derived copper (OD-Cu) has been shown to favor C2 and C3 products in electrochemical CO reduction at lower overpotentials than polycrystalline copper (Cu-poly). Despite numerous studies and proposed mechanisms, the exact nature of the active phase is still a topic of discussion. In this work, we employ operando attenuated total reflection surface enhanced infrared absorption spectroscopy (ATR-SEIRAS) to investigate different sites available on Cu-poly and OD-Cu surfaces using CO as a probe molecule. We identify a CO adsorption band on OD-Cu at 2058 cm–1 that is different from those on Cu-poly but resembles CO bound to the Cu(100) facet. In accordance with reactivity studies, we propose that this band corresponding to distinct CO binding sites is responsible for OD-Cu’s enhanced CO reduction activity.
Journal Title: ACS Catalysis
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!