Photo from wikipedia
Enantiomers, chiral isomers with opposite chirality, typically demonstrate differences in their pharmacological activity, metabolism, and toxicity. However, direct discrimination between enantiomers is challenging due to their similar physiochemical properties. Following… Click to show full abstract
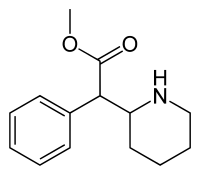
Enantiomers, chiral isomers with opposite chirality, typically demonstrate differences in their pharmacological activity, metabolism, and toxicity. However, direct discrimination between enantiomers is challenging due to their similar physiochemical properties. Following the strategy of programmable nanoreactors for stochastic sensing (PNRSS), introduction of phenylboronic acid (PBA) to a Mycobacterium smegmatis porin A (MspA) assists in the identification of the enantiomers of norepinephrine and epinephrine. Using a machine learning algorithm, identification of the enantiomers has been achieved with an accuracy of 98.2%. The enantiomeric excess (ee) of a mixture of enantiomeric catecholamines was measured to determine the enantiomeric purity. This sensing strategy is a faster method for the determination of ee values than liquid chromatography-mass spectrometry and is useful as a quality control in the industrial production of enantiomeric drugs.
Journal Title: ACS nano
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!