Photo from wikipedia
Polymerization of allyl ether monomers has previously been considered a free-radical addition polymerization mechanism, but it is difficult to achieve because of the high electron density of their double bond.… Click to show full abstract
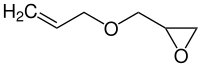
Polymerization of allyl ether monomers has previously been considered a free-radical addition polymerization mechanism, but it is difficult to achieve because of the high electron density of their double bond. To interpret the mechanism of photopolymerization, we therefore proposed a radical-mediated cyclization (RMC) reaction, which has been validated by results from quantum chemistry calculations and real-time infrared observation. Our RMC reaction begins with the radical abstracting one allylic hydrogen atom from the methylene group of allyl ether to generate an allyl ether radical with a delocalized π33 bond. Then, the radical reacts with the double bond of a second allyl ether molecule to form a five-membered cyclopentane-like ring (CP) radical. The CP radical abstracts a hydrogen atom from a third ether molecule. At last, a new allyl ether radical is generated and the next circulation as chain propagation begins. The distortion/interaction model was employed to explore the transient state of reaction, and real-time infrared was chosen to clarify the RMC reaction mechanism initiated by different photoinitiators. These results demonstrated that the RMC mechanism can give new insights into these fundamental processes.
Journal Title: ACS Omega
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!