Photo from wikipedia
Malic acid derivatives from camu-camu (Myrciaria dubia) fruit exhibited a strong in vitro inhibitory activity toward pancreatic α-amylase and α-glucosidase enzymes. During a bioguided chromatographic fractionation process of the whole… Click to show full abstract
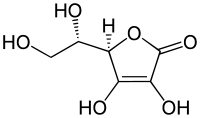
Malic acid derivatives from camu-camu (Myrciaria dubia) fruit exhibited a strong in vitro inhibitory activity toward pancreatic α-amylase and α-glucosidase enzymes. During a bioguided chromatographic fractionation process of the whole fruit (pulp and peelings) polar extract, isomers (S)-4-butoxy-2-hydroxy-4-oxobutanoic acid (1) and (S)-4-butoxy-3-hydroxy-4-oxobutanoic acid (2) (84:16) were isolated and identified as a potent inhibitor of α-amylase (IC50= 11.69 ± 1.75 μg/mL) and α-glucosidase (IC50 = 102.69 ± 4.16 μg/mL). The chemical structures were confirmed by HPLC-ESIMS and 1H and 13C NMR (one- and two-dimensional) analyses. The structure-based virtual screening demonstrated that the aliphatic moiety plays a significant role in the binding mode of the test alkyl malate esters. Compound 1 exhibited the best interaction profile to bind both enzymes, having key structural features to form relevant contacts by involving adequate enzyme–ligand complex stabilization and compactness over time.
Journal Title: ACS Omega
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!