Photo from wikipedia
Poly(ethylene terephthalate) (PET) and phthalate esters (PAEs) are used extensively as plastics and plasticizers. Enzymatic degradation of PET and PAEs has drawn great attention in recent years; however, evolution of… Click to show full abstract
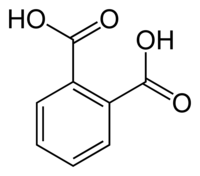
Poly(ethylene terephthalate) (PET) and phthalate esters (PAEs) are used extensively as plastics and plasticizers. Enzymatic degradation of PET and PAEs has drawn great attention in recent years; however, evolution of PET- and PAE-degrading enzymes is still a big challenge, partly because of the lack of an effective screening method to detect phthalic acid (PA) and terephthalic acid (TPA), which are the main hydrolysis products of PAEs and PET. Here, by directed evolution of a promiscuous transcription factor, XylS from Pseudomonas putida, we created two novel variants, XylS-K38R-L224Q and XylS-W88C-L224Q, that are able to bind PA and TPA and activate the downstream expression of a fluorescent reporter protein. Based on these elements, whole-cell biosensors were constructed, which enabled the fluorimetric detection of as little as 10 μM PA or TPA. A PAE hydrolase, GoEst15, was preliminarily engineered using this new biosensor, yielding a mutant GoEst15-V3 whose activity toward dibutyl phthalate (DBP) and p-nitrophenyl butyrate was enhanced 2.0- and 2.5-fold, respectively. It was shown that 96.5% DBP (5 mM) was degraded by GoEst15-V3 in 60 min, while the wild-type enzyme degraded only 55% DBP. This study provides an effective screening tool for directed evolution of PAE-/PET-degrading enzymes.
Journal Title: ACS synthetic biology
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!