Photo from wikipedia
Precisely activating chemotherapeutic prodrugs in a tumor-selective manner is an ideal way to cure cancers without causing systemic toxicities. Although many efforts have been made, developing spatiotemporally controllable activation methods… Click to show full abstract
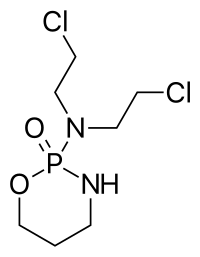
Precisely activating chemotherapeutic prodrugs in a tumor-selective manner is an ideal way to cure cancers without causing systemic toxicities. Although many efforts have been made, developing spatiotemporally controllable activation methods is still an unmet challenge. Here, we report a novel prodrug activation strategy using radiotherapy (X-ray). Due to its precision and deep tissue penetration, X-ray matches the need for altering molecules in tumors through water radiolysis. We first demonstrated that N-oxides can be effectively reduced by hydrated electrons (e-aq) generated from radiation both in tubes and living cells. A screening is performed to investigate the structure-reduction relationship and mechanism of the e-aq-mediated reductions. We then apply the strategy to activate N-oxide prodrugs. The anticancer drug camptothecin (CPT)-based N-oxide prodrug shows a remarkable anticancer effect upon activation by radiotherapy. This radiation-induced in vivo chemistry may enable versatile designs of radiotherapy-activated prodrugs, which are of remarkable clinical relevance, as over 50% of cancer patients take radiotherapy.
Journal Title: Journal of the American Chemical Society
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!