Photo from wikipedia
Next-generation sequencing (NGS) discloses nucleotide changes in the genome. Mutations at splicing regulatory elements are expected to cause splicing errors, such as exon skipping, cryptic splice site activation, partial exon… Click to show full abstract
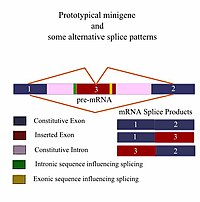
Next-generation sequencing (NGS) discloses nucleotide changes in the genome. Mutations at splicing regulatory elements are expected to cause splicing errors, such as exon skipping, cryptic splice site activation, partial exon loss or intron retention. In dystrophinopathy patients, prediction of splicing outcomes is essential to determine the phenotype: either severe Duchenne or mild Becker muscular dystrophy, based on the reading frame rule. In a Vietnamese patient, NGS identified a c.9361+1G>A mutation in the dystrophin gene and an additional DNA variation of A>G at +117 bases in intron 64. To ascertain the consequences of these DNA changes on dystrophin splicing, minigene constructs were prepared inserting dystrophin exon 64 plus various lengths of intron 64. Exon 64 skipping was observed in the minigene construct with 160 nucleotide (nt) of intron 64 sequence with both c.9361+1A and +117G. In contrast, minigene constructs with larger flanking intronic domains resulted in cryptic splice site activation rather than exon skipping. Meanwhile, the cryptic splice site activation was induced even in +117G when intron 64 was elongated to 272 nt and longer. It was expected that cryptic splice site activation is an in vivo splicing outcome.
Journal Title: Journal of Human Genetics
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!