Photo from wikipedia
The bidentate N,O-ligands phenol-pyrazole (HL1), naphthol-pyrazole (HL2) and the commercially available ligand 5-methylphenol-benzotriazole (HL3) were used for the synthesis of novel iron(iii) complexes. The mononuclear iron complexes [FeCl(L1)2] (1), [FeCl(L2)2]… Click to show full abstract
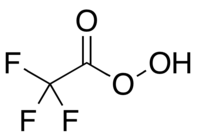
The bidentate N,O-ligands phenol-pyrazole (HL1), naphthol-pyrazole (HL2) and the commercially available ligand 5-methylphenol-benzotriazole (HL3) were used for the synthesis of novel iron(iii) complexes. The mononuclear iron complexes [FeCl(L1)2] (1), [FeCl(L2)2] (2) and [FeCl(L3)2] (3) are stable to air and moisture, both in the solid state as well as in solution, while the dinuclear, μ-oxido bridged complex [{Fe(L1)2}2(μ-O)] (1a) is air sensitive. All four complexes 1, 2, 3 and 1a were investigated for their catalytic activity in the direct one-pot oxidation of primary alcohols to carbonic acids with 30% aq. hydrogen peroxide (H2O2) as the oxidation agent. The activity in oxidation reactions of the isolated, mononuclear complexes 1-3 was further compared to their in situ prepared analogues IS1-3. Experimentally obtained results indicate a tendency of higher activity for the oxidation of primary alcohols for the in situ prepared complexes. In conclusion, the oxidation of aromatic primary alcohols to carboxylic acids using isolated iron(iii) complexes and in situ generated complexes in the presence of H2O2 results in good to high yields. The reaction is straight-forward, clean and generates water as the only by-product.
Journal Title: Dalton transactions
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!