Photo from wikipedia
A mild, selective and redox-neutral Cp*Ir(III)- and Cp*Rh(III)-catalyzed C-H activation/annulation of salicylaldehydes with fluorovinyl tosylates is reported. The use of monofluorovinyl tosylate favors the synthesis of C2- and C3-substitution-free chromones… Click to show full abstract
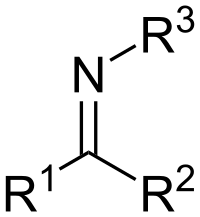
A mild, selective and redox-neutral Cp*Ir(III)- and Cp*Rh(III)-catalyzed C-H activation/annulation of salicylaldehydes with fluorovinyl tosylates is reported. The use of monofluorovinyl tosylate favors the synthesis of C2- and C3-substitution-free chromones via C-H activation/β-F elimination/annulation, whereas difluorovinyl tosylate leads to the construction of C2-fluoroalkoxy chromones. Mild reaction conditions and good functional-group tolerance were observed. Further functionalization of the resulting chromones via halogenation, alkynylation, alkylation and hydrocyanation was successfully realized.
Journal Title: Chemical communications
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!