Photo from wikipedia
Metal–N–C-based catalysts prepared by pyrolysis are frequently used in the oxygen reduction reaction (ORR). Zeolitic imidazolate frameworks (ZIFs), a type of metal organic framework (MOF), are selected as precursors due… Click to show full abstract
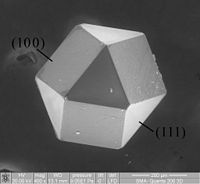
Metal–N–C-based catalysts prepared by pyrolysis are frequently used in the oxygen reduction reaction (ORR). Zeolitic imidazolate frameworks (ZIFs), a type of metal organic framework (MOF), are selected as precursors due to their special structure and proper pore sizes. A series of Fe–N–C catalysts with different concentrations of 2-methylimidazole were prepared with a simple solvothermal-pyrolysis method, and the transformation productivity, morphology and ORR activity were investigated. It was found that the Fe–N–C catalyst with a 2-methylimidazole concentration of 0.53 mol L−1 had the best performance. In 0.1 M KOH solution, the half-wave potential was 0.852 V (vs. RHE), with the highest electrochemically active surface area (ECSA) of 94.1 cm2, and the ORR reaction was dominated by a 4-electron process. The current only decreased by 10.5% after 50 000 s of chronoamperometry (CA), while the half-wave potential only decreased 20 mV in 3 M methanol. Additionally, this catalyst cannot be poisoned by Cl− and SO32− ions in the ORR process. Finally, some typical ions including SCN−, Fe(CN)63− and Fe(CN)64− were used to inhibit the active sites, and it was determined that Fe(ii) is the real active species. The series of synthesis and testing experiments has significance in guiding optimization of the synthesis conditions and analysis of the mechanism of active sites in Fe–N–C materials.
Journal Title: RSC Advances
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!