Photo from wikipedia
A series of poly(hexamethylene 2,5-furandicarboxylate-co-2,6-naphthalate) copolyesters were synthesized using various amounts of poly(hexylene 2,5-furandicarboxylate) (PHF) and poly(hexylene 2,6-naphthalate) (PHN) via melt polymerization. The effects of introducing 2,6-naphthalene dicarboxylic acid (NDCA)… Click to show full abstract
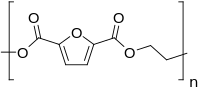
A series of poly(hexamethylene 2,5-furandicarboxylate-co-2,6-naphthalate) copolyesters were synthesized using various amounts of poly(hexylene 2,5-furandicarboxylate) (PHF) and poly(hexylene 2,6-naphthalate) (PHN) via melt polymerization. The effects of introducing 2,6-naphthalene dicarboxylic acid (NDCA) on the thermal, mechanical, and gas-barrier properties were investigated. When the NDCA content was less than 30 mol%, the temperatures of crystallization (Tc) and melting (Tm) decreased as the amount of NDCA was increased owing to disturbance of the polymer-chain regularity. When the NDCA content was above 50 mol%, the Tc and Tm of the materials increased as the NDCA content was increased, showing that the dominant crystallization behavior varied from 2,5-furandicarboxylic acid to NDCA. Hence, the glass transition temperature (Tg) increased as the NDCA content was increased, which was attributed to the incorporation of NDCA with a more rigid naphthalate structure compared with the furan ring. The gas-barrier properties of the samples were observed to improve with the introduction of NDCA; this tendency could be explained by the β-relaxation behavior and free volume values of the samples in the amorphous state. The activation energy (Ea) of β-relaxation increased with the NDCA content, indicating that higher amounts of energy were needed to trigger the onset of long-range molecular motions. Free-volume calculations of the polymer structure showed that the introduction of NDCA hindered the space for gas penetration. For these reasons, the gas-barrier properties were improved and evaluated.
Journal Title: Soft matter
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!