Photo from wikipedia
Selective hydrogenation of dimethyl terephthalate (DMT) is an ideal way to prepare 1,4-cyclohexane dicarboxylate (DMCD), an important intermediate and monomer. Even though noble metal-based catalysts (e.g., Ru, Pd) have been… Click to show full abstract
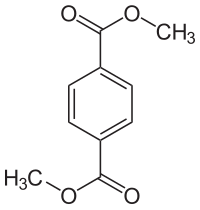
Selective hydrogenation of dimethyl terephthalate (DMT) is an ideal way to prepare 1,4-cyclohexane dicarboxylate (DMCD), an important intermediate and monomer. Even though noble metal-based catalysts (e.g., Ru, Pd) have been developed for selective hydrogenation of DMT, the use of non-precious Ni catalysts to achieve high activity and selectivity is still challenging. In this study, we present that only 0.5 wt% of KF by post-impregnated doping can significantly improve the performance of Ni/SiO2 catalysts (83% vs. 96% selectivity; 41% vs. 95% conversion). The selectivity of DMCD can be up to 97%, which is the highest reported over Ni catalysts. The boosting effect of KF modification might be due to higher amounts of Ni(0) species and lower amounts of moderate acidic sites, which are beneficial for selective hydrogenation of phenyl rings over hydrogenolysis of ester groups.
Journal Title: RSC Advances
Year Published: 2023
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!