Photo from wikipedia
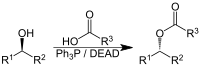
The Mitsunobu reaction has emerged as an important alternative for the preparation of synthetic 2′-deoxynucleosides, which have various biological and biotechnological applications. In this work, the Mitsunobu-based synthesis of 2′-deoxynucleosides… Click to show full abstract
The Mitsunobu reaction has emerged as an important alternative for the preparation of synthetic 2′-deoxynucleosides, which have various biological and biotechnological applications. In this work, the Mitsunobu-based synthesis of 2′-deoxynucleosides was systematically studied. The effect of phosphine, azodicarbonyl reagent, and solvent on the product yield and α/β ratio was investigated, and the highest yield and β-selectivity were obtained using ( n -Bu) 3 P and 1,1′-(azodicarbonyl)dipiperidine in DMF. The reaction was successfully applied to various nucleobase analogues.
Journal Title: Synlett
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!