Photo from wikipedia
Significance Despite being one of the first protein kinases discovered, cyclic guanosine-3′,5′-monophosphate (cGMP)-dependent protein kinase (PKG) has not been successfully crystallized previously, leaving many unanswered questions about its mechanism of… Click to show full abstract
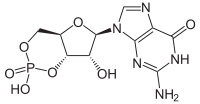
Significance Despite being one of the first protein kinases discovered, cyclic guanosine-3′,5′-monophosphate (cGMP)-dependent protein kinase (PKG) has not been successfully crystallized previously, leaving many unanswered questions about its mechanism of activation. We report herein the structure of cGMP-free PKG from Plasmodium parasites, the causative agents of malaria, one of the world’s deadliest infectious diseases. The structures, combined with data from biochemical and biophysical experiments, provide insight into a mechanism of activation that involves previously unpredicted interdomain communication via a structural relay system. In addition to the full structure of PKG, our work contributes important functional information for a key antimalarial drug target. The cyclic guanosine-3′,5′-monophosphate (cGMP)-dependent protein kinase (PKG) was identified >25 y ago; however, efforts to obtain a structure of the entire PKG enzyme or catalytic domain from any species have failed. In malaria parasites, cooperative activation of PKG triggers crucial developmental transitions throughout the complex life cycle. We have determined the cGMP-free crystallographic structures of PKG from Plasmodium falciparum and Plasmodium vivax, revealing how key structural components, including an N-terminal autoinhibitory segment (AIS), four predicted cyclic nucleotide-binding domains (CNBs), and a kinase domain (KD), are arranged when the enzyme is inactive. The four CNBs and the KD are in a pentagonal configuration, with the AIS docked in the substrate site of the KD in a swapped-domain dimeric arrangement. We show that although the protein is predominantly a monomer (the dimer is unlikely to be representative of the physiological form), the binding of the AIS is necessary to keep Plasmodium PKG inactive. A major feature is a helix serving the dual role of the N-terminal helix of the KD as well as the capping helix of the neighboring CNB. A network of connecting helices between neighboring CNBs contributes to maintaining the kinase in its inactive conformation. We propose a scheme in which cooperative binding of cGMP, beginning at the CNB closest to the KD, transmits conformational changes around the pentagonal molecule in a structural relay mechanism, enabling PKG to orchestrate rapid, highly regulated developmental switches in response to dynamic modulation of cGMP levels in the parasite.
Journal Title: Proceedings of the National Academy of Sciences of the United States of America
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!