Photo from wikipedia
Significance Hemagglutinin (HA) is the receptor binding and membrane fusion glycoprotein of influenza virus. Like other virus fusion glycoproteins such as those of HIV and Ebola, HA is synthesized as… Click to show full abstract
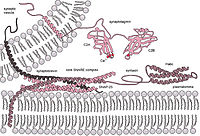
Significance Hemagglutinin (HA) is the receptor binding and membrane fusion glycoprotein of influenza virus. Like other virus fusion glycoproteins such as those of HIV and Ebola, HA is synthesized as a precursor (HA0) that requires cleavage for fusion activity and, for influenza, exposure to low pH. Studies by X-ray and cryogenic electron microscopy (cryo-EM) have characterized conformational changes in HA that occur at membrane fusion pH. Here, using cryo-EM, we report that there are extensive changes to the structure of HA0 at low pH but that, unlike the changes in HA, the changes are reversible on return to neutral pH. The low-pH structure of HA0 is considered an indicator of potential intermediates in the conformational changes in HA at fusion pH.
Journal Title: Proceedings of the National Academy of Sciences of the United States of America
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!