Photo from wikipedia
Significance Within cells, enzymes and cofactors catalyzing multistep processes (cascades) are often confined together—either in enclosures (e.g., organelles) or via physical association (metabolons). Nanoconfinement, offering potential general advantages for catalysis,… Click to show full abstract
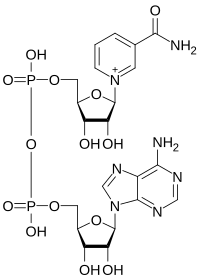
Significance Within cells, enzymes and cofactors catalyzing multistep processes (cascades) are often confined together—either in enclosures (e.g., organelles) or via physical association (metabolons). Nanoconfinement, offering potential general advantages for catalysis, can also be achieved by loading enzymes and their exchangeable cofactors into a porous, electrically conducting inorganic material, thereby enabling catalysis to be channeled, energized, and investigated electrochemically. Such nanoconfinement enables a cascade comprising electroactive ferredoxin NADP+ reductase and isocitrate dehydrogenase to be active for days, catalyzing exhaustive oxidation of bulk isocitrate by recycling trapped NADP(H) carried in on isocitrate dehydrogenase. Nanoconfinement massively increases the efficiency of cofactor-dependent cascade catalysis and has conceptual relevance for prebiotic evolution where complex organic molecules might have formed in gaps and cracks of minerals.
Journal Title: Proceedings of the National Academy of Sciences of the United States of America
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!