Photo from wikipedia
Significance Human leukocyte antigens (HLAs) present peptides to T cells, thereby allowing them to recognize pathogen-infected and cancer cells. Conventionally, it was thought that peptides bind to HLA molecules in… Click to show full abstract
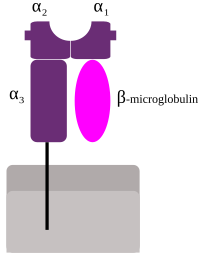
Significance Human leukocyte antigens (HLAs) present peptides to T cells, thereby allowing them to recognize pathogen-infected and cancer cells. Conventionally, it was thought that peptides bind to HLA molecules in a conserved N- to C-terminal orientation. Here, we demonstrate that peptides in HLA-DP can also bind in a reverse C- to N-terminal orientation and show recognition of such reverse peptides by virus-specific human T cells from the memory repertoire. We provide the molecular basis for reverse binding by a high-resolution HLA–peptide crystal structure. Our findings have impact on our basic understanding of how peptides bind to HLA molecules and expand the identification of novel peptides recognized by T cells, which will help improving treatment of viral infections and cancers.
Journal Title: Proceedings of the National Academy of Sciences of the United States of America
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!