Photo from wikipedia
ABSTRACT The dielectric relaxation study for the hydrogen bonded binary system of polar liquid ethyl acetate in 1-butanol and 1-pentanol, has been carried out at 11 concentrations over the frequency… Click to show full abstract
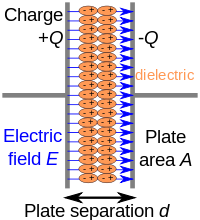
ABSTRACT The dielectric relaxation study for the hydrogen bonded binary system of polar liquid ethyl acetate in 1-butanol and 1-pentanol, has been carried out at 11 concentrations over the frequency range of 10 MHz to 20 GHz at 30°C using TDR. The least squares fit method has been used to obtain the static dielectric constant (ϵ0) and relaxation time (τ). By using dielectric parameters the excess permittivity (ϵE), excess inverses relaxation time (1/τ)E, Kirkwood Correlation factor (g) are also obtained to explore the hydrogen-bonded hetero-molecular interactions. Conformational analysis of the hydrogen bond between the two systems is supported by the FTIR spectra.
Journal Title: Ferroelectrics
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!