Photo from wikipedia
Abstract A one-pot method for the simple, efficient, and environmentally benign protocol for the synthesis of Spiro-Indoline/Indene derivatives containing Aza indole moiety as a side chain is outlined. The 1,3-dipolar… Click to show full abstract
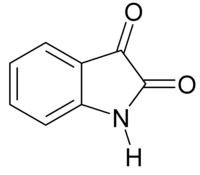
Abstract A one-pot method for the simple, efficient, and environmentally benign protocol for the synthesis of Spiro-Indoline/Indene derivatives containing Aza indole moiety as a side chain is outlined. The 1,3-dipolar cycloaddition reaction of azomethine ylides derived from ethynyl Azaindole under mild conditions is accomplished. The low cost, easy availability of the starting material, and simple reaction conditions suggest the possible use of the present method for large-scale preparations. Graphical Abstract
Journal Title: Synthetic Communications
Year Published: 2018
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!