Photo from wikipedia
Abstract Gonadotropin-releasing hormone (GnRH) agonists are peptides consisting of nine or ten amino acid residues. GnRH agonists have been applied in the therapy of sexual hormone disorders like prostate cancer,… Click to show full abstract
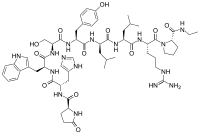
Abstract Gonadotropin-releasing hormone (GnRH) agonists are peptides consisting of nine or ten amino acid residues. GnRH agonists have been applied in the therapy of sexual hormone disorders like prostate cancer, endometriosis, uterine myoma, central precious puberty, and in-vitro fertility. Treatment is achieved by continuous hormone intake and long-term agonists administration, which is usually associated with poor patient compliance. Because GnRH agonists that are administered with the parenteral route are broken down by peptidase, their half-life is short. As a result, developing sustained release for the drug delivery system is significant. Even though some drugs have been successfully delivered with long-acting release microspheres and approved by the Food and Drug Administration (FDA), some challenges remain. This review highlighted current approaches to encapsulate GnRH agonists into delivery systems and strategies encountered during the loading process. Moreover, the following sections provide strategies to improve the release profile, and animal and human studies were summarised.
Journal Title: Journal of Microencapsulation
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!