Photo from wikipedia
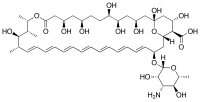
Abstract AmBisome (liposomal amphotericin B) is among the earliest approved liposomal therapeutics, and has been in commercial use since the early 1990s. This review provides examples of non-clinical, regulatory, clinical… Click to show full abstract
Abstract AmBisome (liposomal amphotericin B) is among the earliest approved liposomal therapeutics, and has been in commercial use since the early 1990s. This review provides examples of non-clinical, regulatory, clinical label expansion, adverse event management, and supply chain control reflecting the real world challenges of a commercial liposomal therapeutic. We review examples of post-approval clinical development in severe lung infections, development of US and European guidance documents around liposomal therapeutics, the creation of a suitable placebo for blinded clinical trials, response to findings of a possible new category of adverse event (what turned out to be pseudohyperphosphatemia), challenges in handling the finished product in a setting with high risk of exposure of the product to temperatures outside of the established label storage conditions, and elements of continuingly increased aseptic processing requirements for manufacturing.
Journal Title: Journal of Liposome Research
Year Published: 2017
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!