Photo from wikipedia
ABSTRACT The STX17-SNAP29-VAMP8 SNARE complex mediates autophagosome-lysosome fusion. Our recent study showed that MTOR directly phosphorylates VAMP8ʹs T48 residue in nutrient-rich conditions. Phosphorylated VAMP8 inhibits autophagosome-lysosome fusion by blocking STX17-SNAP29-VAMP8… Click to show full abstract
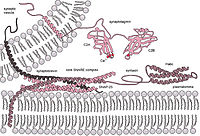
ABSTRACT The STX17-SNAP29-VAMP8 SNARE complex mediates autophagosome-lysosome fusion. Our recent study showed that MTOR directly phosphorylates VAMP8ʹs T48 residue in nutrient-rich conditions. Phosphorylated VAMP8 inhibits autophagosome-lysosome fusion by blocking STX17-SNAP29-VAMP8 SNARE complex formation. Our study also showed that SCFD1 is a previously unrecognized macroautophagy/autophagy regulatory protein, which can be recruited by VAMP8 (in its non-phosphorylated form) to autolysosomes, where it promotes STX17-SNAP29-VAMP8 complex assembly – and consequently promotes autophagosome-lysosome fusion. Moreover, we observed that mice harboring a phosphomimic VAMP8 variant accumulate aberrantly high lipid levels in their livers. VAMP8 phosphorylation can disrupt autophagosome-lysosome fusion in the liver and thereby dysregulate lipid metabolism. Beyond providing insights into the molecular mechanisms of autophagosome maturation, our study suggests that modulating autophagic SNARE function may help treat liver lipid disorders.
Journal Title: Autophagy
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!