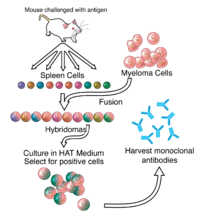
Photo from wikipedia
Throughout history, pandemics like the Black Death or the Spanish Flue have periodically wiped out large parts of populations. Today, history repeats itself with the on-going SARSCoV-2 pandemic, which manifests… Click to show full abstract
Throughout history, pandemics like the Black Death or the Spanish Flue have periodically wiped out large parts of populations. Today, history repeats itself with the on-going SARSCoV-2 pandemic, which manifests that pandemics still hold the potential to cause devastating impact to global health and economy. To prevent severe illness and death, epidemics have been fought with polyclonal antibodies derived from immunized animals, since this technology was invented by Behring and Kitasato in 1890 [1]. Since then, major advances have occurred in the field of immunotherapy, enabled particularly by the advent of recombinant DNA technology and a range of discovery methodologies that allow for the generation and manufacture of (human) monoclonal antibodies and antibody fragments [2]. These advances strengthen our ability to react to infectious diseases with pandemic potential and have already been put to utility in a number of cases [3]. As an example, during the 2014–2016 Ebola outbreak in West Africa, a mixture of three monoclonal antibodies (ZMapp) originating from two prior antibody mixtures (MB003 and Zmab) were combined and rapidly used for treatment of Ebola infected patients despite lacking prior clinical trials to prove safety and efficacy [4]. The antibody mixture was shown to be beneficial to the patients, although it did not reach statistical significance compared to standard of care [5]. In addition, therapeutic monoclonal antibodies have been, or are currently being developed for H1N1 [6], SARS, MERS [7], and SARS-CoV-2 [8]. While vaccine development is supreme to reduce mortality in a pandemic situation, and convalescent plasma or small molecules sometimes can be used for treatment, an urgent need also exists for fast development of therapeutic monoclonal antibodies as a complement. These antibodies can be administered to groups responding weakly to vaccination, such as immunocompromised patients, or prophylactically as a first line of defense for people at high risk of being infected and/ or becoming severely ill. In addition, if prompt testing to detect infected people is in place, an opportunity may exist to administer antibodies as a precaution and treatment before clinical manifestations occur, thereby possibly reducing the severity of an infection. 2. Challenges
Journal Title: Expert Opinion on Drug Discovery
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!