Photo from wikipedia
Nickel-silver core-shell (Ni@Ag) nanoparticles (NPs) were formed in a two-step process: (1) the formation of a dispersion of Ni NPs; and (2) the transmetalation (galvanic displacement) reaction, where the surface… Click to show full abstract
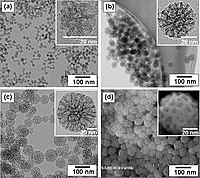
Nickel-silver core-shell (Ni@Ag) nanoparticles (NPs) were formed in a two-step process: (1) the formation of a dispersion of Ni NPs; and (2) the transmetalation (galvanic displacement) reaction, where the surface of the Ni NPs acted as the reducing agent of Ag ions. Ni NPs were synthesized by the 'wet' chemical method, i.e., by the reduction of metal ions by using NaBH4 as the reducing agent. The influence of the concentration of polymeric stabilizer, reducing agent and Ag precursor on the properties of synthesized NPs was evaluated. In the optimal condition of synthesis, Ni@Ag NPs with about 50 and 210 nm-diameter Ni core coated with a thin (∼10-20 nm) Ag shell, were obtained. Finally, the stability of the synthesized spherical-shaped Ni@Ag NPs was tested and the results indicate long-term stability against aggregation and Ni oxidation. Thus, the resulting NPs are promising candidates for application in electronic devices, e.g., as components of conductive inks or pastes.
Journal Title: Nanotechnology
Year Published: 2019
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!