Photo from wikipedia
An ethanol solution of nitrate combustion process for the fabrication of magnetic Ni0.3Co0.2Zn0.5Fe2O4 nanoparticles was introduced. And the Ni0.3Co0.2Zn0.5Fe2O4 nanoparticles fabricated at 500 °C for 2 h were characterized by… Click to show full abstract
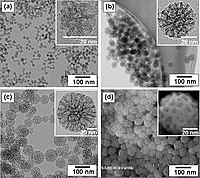
An ethanol solution of nitrate combustion process for the fabrication of magnetic Ni0.3Co0.2Zn0.5Fe2O4 nanoparticles was introduced. And the Ni0.3Co0.2Zn0.5Fe2O4 nanoparticles fabricated at 500 °C for 2 h were characterized by means of the transmission electron microscopy (TEM), the vibrating sample magnetometer (VSM), the scanning electron microscopy (SEM), and the x-ray diffraction (XRD). The magnetic Ni0.3Co0.2Zn0.5Fe2O4 nanoparticles were useful to remove reactive red 2BF (RR-2BF) from wastewater, and the adsorption mechanism of RR-2BF onto magnetic Ni0.3Co0.2Zn0.5Fe2O4 nanoparticles was explored. The experimental results revealed that Temkin isotherm model and the pseudo-second kinetics model matched well with adsorption process, which indicated that there was monolayer and multilayer adsorption in the adsorption behavior of RR-2BF onto magnetic Ni0.3Co0.2Zn0.5Fe2O4 nanoparticles. While, the effects of the adsorbent dosage and pH value of solution on the adsorption process were investigated, and it was found that with the increase of them, the adsorbances of RR-2BF onto Ni0.3Co0.2Zn0.5Fe2O4 nanoparticles decreased. The maximum adsorption capacity of Ni0.3Co0.2Zn0.5Fe2O4 was about 138 mg g−1, and the adsorbance amounted to 75% of the initial one after 8 recycles.
Journal Title: Materials Research Express
Year Published: 2021
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!