Photo from wikipedia
Abstract Introduction Anti-staphylococcal penicillins (ASPs) are among the most commonly prescribed antibiotics in children and are associated with a risk of drug-induced liver injury (DILI). Despite the frequent use of… Click to show full abstract
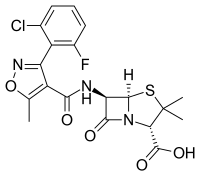
Abstract Introduction Anti-staphylococcal penicillins (ASPs) are among the most commonly prescribed antibiotics in children and are associated with a risk of drug-induced liver injury (DILI). Despite the frequent use of ASPs in children, there is no consensus on whether liver function tests (LFTs) should be routinely monitored during treatment. Objectives To review the literature on the frequency of ASP-related DILI in children to determine the incidence, risk factors and outcomes of hepatotoxicity. Methods PubMed, MEDLINE and Embase were searched in January 2022 for original studies of children who received cloxacillin, dicloxacillin, flucloxacillin, methicillin, nafcillin or oxacillin that included ≥10 children aged up to 18 years, and presented data on the incidence of DILI in children exposed to ASPs. Results Overall, two studies of oral flucloxacillin, two of intravenous (IV) methicillin, three of IV nafcillin and four of IV oxacillin were included. The mean onset of DILI ranged between 7.0 and 19.0 days following commencement of antibiotic treatment and all episodes resolved between 14.2 and 16.0 days after drug discontinuation, with no specific treatment required. This review found that the incidence of DILI in children was 1 in 50 000 for oral flucloxacillin and ranged from 1 in 3 to 13 for IV oxacillin, methicillin and nafcillin. Conclusions This review found that routine LFT monitoring is not required in children receiving low dose oral flucloxacillin in a primary care setting, although pharmacovigilance is critical. For IV preparations, the existing data support routine LFT monitoring in those receiving treatment for at least 7 days.
Journal Title: Journal of Antimicrobial Chemotherapy
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!