Photo from wikipedia
Presented is the analysis of four cannabinoid-based products. These products were part of a case involving visual and auditory hallucinations that precipitated the commission of a felony and subsequent arrest.… Click to show full abstract
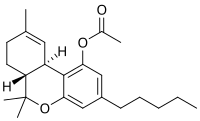
Presented is the analysis of four cannabinoid-based products. These products were part of a case involving visual and auditory hallucinations that precipitated the commission of a felony and subsequent arrest. The products were labelled to contain Δ8-tetrahydrocannabinol (Δ8-THC) or THC acetate (THC-O-A). Primary reference materials were not available for Δ8-THC-O-A, Δ1°-THC-O-A, cannabidiol di-acetate (CBD-di-O-A), or respective deuterated internal standards. THC-O-A and CBD-di-O-A standards were prepared by derivatizing Δ8-THC, Δ9-THC, Δ1°-THC, CBD, Δ9-THC-d3, and CBD-d3 using acetic anhydride. The cannabinoid-based products were determined to contain Δ8-THC, Δ8-THC-O-A, Δ9-THC-O-A, and CBD-di-O-A and/or other phytocannabinoids using three different analytical techniques. Direct analysis in real time-time of flight-mass spectrometry was used for identifying exact masses. A gas chromatograph-mass spectrometer was used for identification of compounds and to quantitate THC-O-As in the products. A liquid chromatograph-tandem mass spectrometer was used to identify and quantitate phytocannabinoids and CBD-di-O-A in the products. To the authors' knowledge, this is the first case report involving the identification of THC-O-As and CBD-di-O-A in commercially available products. Minimal clinical/pharmacological data is available for these emerging synthetic cannabinoids/novel psychoactive substances.
Journal Title: Journal of analytical toxicology
Year Published: 2022
Link to full text (if available)
Share on Social Media: Sign Up to like & get
recommendations!